Dementia Associated with Alzheimer’s Disease Drug Development Surges with 180+ Companies Pushing New Therapeutic Frontiers | DelveInsight
Dementia associated with Alzheimer’s disease is a progressive neurodegenerative condition characterized by memory loss, cognitive decline, and impaired daily functioning. The growing aging population worldwide is leading to a rising prevalence of Alzheimer’s-related dementia, fueling demand for effective diagnostics and disease-modifying therapies.
New York, USA, July 17, 2025 (GLOBE NEWSWIRE) -- Dementia Associated with Alzheimer’s Disease Drug Development Surges with 180+ Companies Pushing New Therapeutic Frontiers | DelveInsight
Dementia associated with Alzheimer’s disease is a progressive neurodegenerative condition characterized by memory loss, cognitive decline, and impaired daily functioning. The growing aging population worldwide is leading to a rising prevalence of Alzheimer’s-related dementia, fueling demand for effective diagnostics and disease-modifying therapies.
DelveInsight’s 'Dementia Associated with Alzheimer’s Disease Pipeline Insight 2025' report provides comprehensive global coverage of pipeline dementia associated with Alzheimer’s disease therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the dementia associated with Alzheimer’s disease pipeline domain.
Key Takeaways from the Dementia Associated with Alzheimer’s Disease Pipeline Report
- DelveInsight’s dementia associated with Alzheimer’s disease pipeline report depicts a robust space with 180+ active players working to develop 200+ pipeline dementia associated with Alzheimer’s disease drugs.
- Key dementia associated with Alzheimer’s disease companies such as BioVie, Bristol Myers Squibb, Cognition Therapeutics, TrueBinding, KeyMed Biosciences, Alzinova, AriBio Co., Ltd., Cognition Therapeutics, AbbVie Inc., Allyx Therapeutics, Inc., Eisai Inc., Shanghai Hengrui Pharmaceutical Co., Ltd., Amylyx Pharmaceuticals Inc., Merry Life Biomedical Company, Praxis Bioresearch, Alector, Inc., Galimedix, MIRAMOON Pharma, NervGen Pharma, Psy Therapeutics, NW PharmaTech, PerioTrap Pharmaceuticals, ADEL, Inc., TauRx Therapeutics Ltd, Eli Lilly and Company, Suven Life Sciences, AB Science, Neurim Pharmaceuticals, Merck Sharp & Dohme, Novartis, Priavoid, Pharmazz, Partner Therapeutics, UCB Biopharma, Longeveron Inc., and others are evaluating new dementia associated with Alzheimer’s disease drugs to improve the treatment landscape.
- Promising pipeline dementia associated with Alzheimer’s disease therapies, such as Bezisterim KarXT, CT-1812, TB 006, CM383, ALZ 101, AR1001, CT1812, ABBV-916, ALX-001, E2814, VT301, AMX0035, TML-6, PRX-P4-003, ADP037-ABC, GAL-201, MP-010, NVG 300, PSY-02, EMCBD-1, S-636, ADEL-Y07, TRx0237, Remternetug, Masupirdine, Masitinib, Piromelatine, MK-1167, Siponimod, PRI-002, PMZ-1620, Sargramostim, Bepranemab, Lomecel-B, and others, are in different phases of Dementia Associated with Alzheimer’s Disease clinical trials.
- In May 2025, Nuravax Inc. was awarded a USD 3 million grant from the National Institutes of Health (NIH) to support Duvax, the first dual-target Alzheimer's vaccine, in its entry into human clinical trials.
- In February 2025, NKGen Biotech, Inc. announced that the US Food and Drug Administration had granted Fast Track designation for the investigation of troculeucel, ex vivo expanded autologous NK cell therapy, for the treatment of moderate Alzheimer’s disease.
- In February 2025, Swedish pharmaceutical company AlzeCure Pharma secured a Eur 2.5m (USD 2.62m) grant from the EU’s European Innovation Council (EIC) to support a Phase IIa trial of NeuroRestore ACD856 for Alzheimer’s disease.
- In April 2025, Biogen announced that the US Food and Drug Administration had granted Fast Track designation to BIIB080, an investigational antisense oligonucleotide (ASO) therapy targeting tau, for the treatment of Alzheimer’s disease.
- In March 2025, Longeveron Inc. announced the positive outcome of a Type B meeting with the US Food and Drug Administration, supporting the advancement of laromestrocel (Lomecel-BTM), a proprietary, scalable, allogeneic, investigational cellular therapy, as a potential treatment for Alzheimer’s disease. The Company and the FDA reached foundational alignment on the overall study design for a proposed single, pivotal, seamless adaptive Phase II/III clinical trial, including proposed AD patient population, proposed placebo control, laromestrocel (Lomecel-BTM) dose selection and frequency, trial duration, and trial endpoints. To accelerate the pathway to potential approval, the FDA agreed to consider a BLA based on positive interim trial results from the planned single study.
- In February 2025, Neuphoria Therapeutics announced that the company was due to receive a USD 15 million milestone payment from Merck, known as MSD outside the United States and Canada. The payment was triggered by the initiation, by Merck, of a Phase II clinical trial to evaluate the safety and efficacy of MK-1167, an α7 nicotinic acetylcholine receptor positive allosteric modulator (PAM), for the treatment of the symptoms of Alzheimer’s disease dementia.
- In February 2025, NKGen Biotech announced that the US Food and Drug Administration has granted Fast Track designation for the investigation of troculeucel, ex vivo expanded autologous NK cell therapy, for the treatment of moderate Alzheimer’s disease.
- In February 2025, NeuroTherapia, Inc., a clinical-stage company focused on developing oral therapies for neurodegenerative diseases, announced it had received approval for its Phase II clinical trial from the European Medicines Agency (EMA). NTRX-07, the company’s lead molecule, will be administered to Alzheimer’s disease (AD) participants for 28 days in this double-masked, randomized clinical trial. In addition to monitoring safety, pharmacokinetics, and standard measures of clinical efficacy (ADAS-cog, MMSA, and Trails Making Test), the trial is also designed to give an indication of target engagement by analyzing various biomarkers of neuroinflammation and neuronal function.
- In January 2025, the US FDA granted Fast Track designation to Posdinemab, a phosphorylated tau-directed monoclonal antibody (mAb) being investigated to treat patients with early Alzheimer’s disease.
- In November 2024, Alector announced results from the INVOKE-2 Phase II clinical trial evaluating the safety and efficacy of AL002 in slowing disease progression in individuals with early Alzheimer’s disease (AD).
- In October 2024, Hoth Therapeutics, Inc. announced the granting of a US patent for its pioneering Alzheimer's treatment, HT-ALZ.
Request a sample and discover the recent advances in dementia associated with Alzheimer’s disease drugs @ Dementia Associated with Alzheimer’s Disease Pipeline Report
The dementia associated with Alzheimer’s disease pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage dementia associated with Alzheimer’s disease drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the dementia associated with Alzheimer’s disease clinical trial landscape.
Dementia Associated with Alzheimer’s Disease Overview
Dementia is a broad term that refers to a marked decline in cognitive abilities that affects a person's daily functioning. Alzheimer’s disease (AD) is the most common form of dementia, responsible for at least two-thirds of cases in people aged 65 and older. AD is a progressive neurodegenerative disorder that begins gradually and leads to worsening impairments in behavior and cognitive skills such as memory, understanding, language, attention, reasoning, and judgment. Although Alzheimer’s itself is not directly fatal, it greatly increases the risk of other complications that can ultimately result in death.
The diagnosis of Alzheimer’s-related dementia usually requires a thorough evaluation, including medical history, cognitive assessments, physical and neurological exams, and sometimes brain imaging. Physicians first exclude other possible causes of cognitive decline, like vitamin deficiencies or thyroid issues. Cognitive tests evaluate memory, problem-solving, and language to identify patterns indicative of Alzheimer’s. Brain scans, such as MRI or CT, help detect structural changes like brain shrinkage. More advanced diagnostic tools, including PET scans or cerebrospinal fluid tests, can reveal specific biomarkers linked to Alzheimer’s, offering stronger confirmation.
Currently, there is no cure for Alzheimer’s disease since brain cell loss cannot be reversed. However, treatments are available to ease symptoms and enhance the quality of life for patients and their caregivers. Cholinesterase inhibitors are medications that help reduce cognitive symptoms such as memory problems, confusion, and impaired judgment by improving communication between brain cells and slowing symptom progression. The three FDA-approved drugs commonly used for Alzheimer’s treatment include donepezil (Aricept) for all stages, and galantamine (Razadyne) and rivastigmine (Exelon) for mild to moderate stages.
Find out more about dementia associated with Alzheimer’s disease drugs @ Dementia Associated with Alzheimer’s Disease Treatment
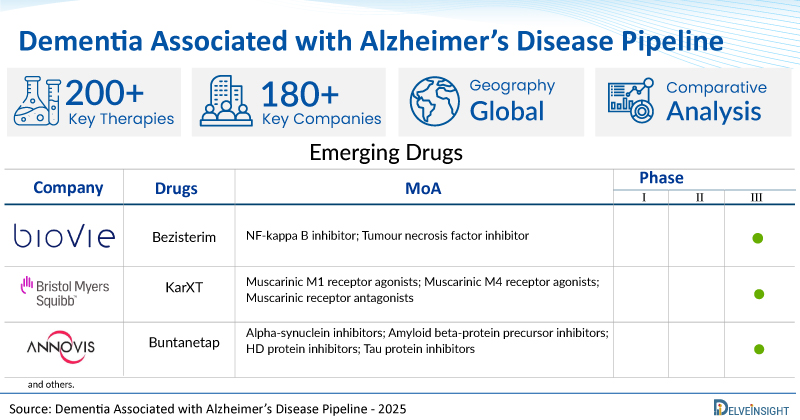
A snapshot of the Pipeline Dementia Associated with Alzheimer’s Disease Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Bezisterim (NE3107) | BioVie | III | NF-kappa B inhibitor; Tumour necrosis factor inhibitor | Oral |
| KarXT | Bristol Myers Squibb | III | Muscarinic M1 receptor agonists; Muscarinic M4 receptor agonists; Muscarinic receptor antagonists | Oral |
| Buntanetap | Annovis Bio | III | Alpha-synuclein inhibitors; Amyloid beta-protein precursor inhibitors; HD protein inhibitors; Tau protein inhibitors | Oral |
| AR1001 | AriBio | III | Type 5 cyclic nucleotide phosphodiesterase inhibitors | Oral |
| CT-1812 | Cognition Therapeutics | II | Sigma-2 receptor antagonists | Oral |
| TW001 | Treeway B.V. | II | Antioxidants; Free radical scavengers | Oral |
| AMX0035 | Amylyx Pharmaceuticals | II | Ammonia scavenger; Histone deacetylase inhibitor; Phosphotransferase inhibitor | Oral |
| CM383 | KeyMed Biosciences | I | Amyloid beta-protein inhibitors | Intravenous |
Learn more about the emerging dementia associated with Alzheimer’s disease therapies @ Dementia Associated with Alzheimer’s Disease Clinical Trials
Dementia Associated with Alzheimer’s Disease Therapeutics Assessment
The dementia associated with Alzheimer’s disease pipeline report proffers an integral view of the emerging dementia associated with Alzheimer’s disease therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Dementia Associated with Alzheimer’s Disease Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Adiponectin stimulants, Interleukin 23 inhibitors, Interleukin 6 inhibitors, Mitogen-activated protein kinase 1 inhibitors, Mitogen-activated protein kinase 3 inhibitors, NF-kappa B inhibitors, Tumour necrosis factor inhibitors, Muscarinic M1 receptor agonists, Muscarinic M4 receptor agonists, Muscarinic receptor antagonists, Microfilament protein modulators, Sigma-2 receptor antagonists, Amyloid beta-protein inhibitors, TREM2 protein-stimulants, Type 5 cyclic nucleotide phosphodiesterase inhibitors, Ammonia scavengers, Histone deacetylase inhibitors, Phosphotransferase inhibitors
- Key Dementia Associated with Alzheimer’s Disease Companies: BioVie, Bristol Myers Squibb, Cognition Therapeutics, TrueBinding, KeyMed Biosciences, Alzinova, AriBio Co., Ltd., Cognition Therapeutics, AbbVie Inc., Allyx Therapeutics, Inc., Eisai Inc., Shanghai Hengrui Pharmaceutical Co., Ltd., Amylyx Pharmaceuticals Inc., Merry Life Biomedical Company, Praxis Bioresearch, Alector, Inc., Galimedix, MIRAMOON Pharma, NervGen Pharma, Psy Therapeutics, NW PharmaTech, PerioTrap Pharmaceuticals, ADEL, Inc., TauRx Therapeutics Ltd, Eli Lilly and Company, Suven Life Sciences, AB Science, Neurim Pharmaceuticals, Merck Sharp & Dohme, Novartis, Priavoid, Pharmazz, Partner Therapeutics, UCB Biopharma, Longeveron Inc., and others.
- Key Dementia Associated with Alzheimer’s Disease Pipeline Therapies: Bezisterim KarXT, CT-1812, TB 006, CM383, ALZ 101, AR1001, CT1812, ABBV-916, ALX-001, E2814, VT301, AMX0035, TML-6, PRX-P4-003, ADP037-ABC, GAL-201, MP-010, NVG 300, PSY-02, EMCBD-1, S-636, ADEL-Y07, TRx0237, Remternetug, Masupirdine, Masitinib, Piromelatine, MK-1167, Siponimod, PRI-002, PMZ-1620, Sargramostim, Bepranemab, Lomecel-B, and others.
Dive deep into rich insights for new dementia associated with Alzheimer’s disease treatments, visit @ Dementia Associated with Alzheimer’s Disease Drugs
Table of Contents
| 1. | Dementia Associated with Alzheimer’s Disease Pipeline Report Introduction |
| 2. | Dementia Associated with Alzheimer’s Disease Pipeline Report Executive Summary |
| 3. | Dementia Associated with Alzheimer’s Disease Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Dementia Associated with Alzheimer’s Disease Clinical Trial Therapeutics |
| 6. | Dementia Associated with Alzheimer’s Disease Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Dementia Associated with Alzheimer’s Disease Pipeline: Late-Stage Products (Phase III) |
| 8. | Dementia Associated with Alzheimer’s Disease Pipeline: Mid-Stage Products (Phase II) |
| 9. | Dementia Associated with Alzheimer’s Disease Pipeline: Early-Stage Products (Phase I) |
| 10. | Dementia Associated with Alzheimer’s Disease Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Dementia Associated with Alzheimer’s Disease Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Dementia Associated with Alzheimer’s Disease Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the dementia associated with Alzheimer’s disease pipeline therapeutics, reach out @ Dementia Associated with Alzheimer’s Disease Therapeutics
Related Reports
Alzheimer's Disease Epidemiology
Alzheimer's Disease Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted Alzheimer’s disease epidemiology in the 7MM, i.e., the United States, EU4 (Germany, Spain, Italy, and France), the United Kingdom, and Japan.
Alzheimer’s Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Alzheimer’s disease companies, including BioVie, AB Science, Cassava Sciences, TauRx Therapeutics, Novo Nordisk, KeifeRx, Eli Lilly, AriBio, Cerecin, Alzheon, Neurim Pharmaceuticals, Syneos Health, Athira Pharma, Annovis Bio, Anavex Life Sciences, AgeneBio, Eisai, among others.
Agitation in Alzheimer’s Disease Market
Agitation in Alzheimer’s Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key agitation in Alzheimer’s disease companies, including Eli Lilly and Co, BioVie Inc., AB Science SA, Annovis Bio Inc., Cognition Therapeutics Inc., Coya Therapeutics Inc., Actinogen Medical Limited, AC Immune SA, Biogen Inc., Longeveron Inc., among others.
Psychosis in Parkinson’s and Alzheimer’s Disease Market
Psychosis in Parkinson’s and Alzheimer’s Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key psychosis in Parkinson’s and Alzheimer’s disease companies, including Sunovion Pharmaceuticals, Karuna Therapeutics, Vanda Pharmaceuticals, Suven Life Sciences, Enterin, Intra-Cellular Therapies, Merck Sharp & Dohme, among others.
Alzheimer’s Disease Diagnostic Market
Alzheimer’s Disease Diagnostic Market Insights, Competitive Landscape and Market Forecast – 2032 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key Alzheimer’s disease diagnostic companies, including F. Hoffmann-La Roche Ltd., General Electric Company, 23andMe, Inc., Lilly, Fujirebio, Siemens Medical Solutions USA, Inc., Diadem srl., Todos Medical, DISCERN™, FUJIFILM Holdings America Corporation, Koninklijke Philips N.V., CANON MEDICAL SYSTEMS EUROPE B.V., Shimzadu Corporation., Laboratory Corporation of America® Holdings, Bruker, Magnetica., IMRIS, Deerfield Imaging, Inc., MR Solutions, Hyperfine, Inc., Neusoft Corporation, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
