GlucoModicum unveils Sofio™, the world’s first needle-free glucose monitor based on magneto-hydrodynamics (MHD)
PRESS RELEASE — FOR IMMEDIATE RELEASE
GlucoModicum unveils Sofio™, the world’s first needle-free glucose monitor based on magneto-hydrodynamics (MHD)
- MHD technology draws micro-sample through skin; glucose is measured using established electrochemical sensor technology
- Single-day sensor system offers periodic and personalized monitoring alternative to continuous glucose monitoring devices
-
Extensive multi-year clinical studies (N>2,000) have demonstrated accuracy, supporting a clear pathway toward CE marking
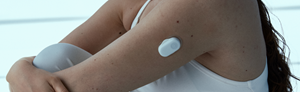
Helsinki, Finland, 18 December - GlucoModicum today introduces Sofio™, a new glucose monitoring device that measures glucose through skin without using needles, supported by extensive multi-year clinical studies.
Sofio uses patented magnetohydrodynamics (MHD) to draw a micro-sample of interstitial fluid through skin, where glucose is measured using established glucose oxidase enzyme chemistry, the same method used in medical-grade glucose meters and CGMs.
“Needle-free glucose monitoring has been one of the most elusive goals in diabetes management for over thirty years,” said Jokke Mäki, CEO of GlucoModicum. “Our MHD-based approach takes a different route, enabling accurate glucose measurement without needles while relying on the established biosensing chemistry used in clinical devices.”
Designed for people who don’t need 24/7 monitoring
Sofio has been developed with the understanding that Type 2 diabetes is not static. Glucose patterns shift with lifestyle, medication, genetics and age. Most people with Type 2 diabetes don’t need a sensor attached to them for weeks. Sofio enables single-day glucose monitoring sessions whenever deeper insights are needed - during dietary or lifestyle changes, medication adjustments, or periodic checkups.
The system includes a durable, rechargeable transmitter (lasting up to two years) and a gentle, replaceable single-day sensor, offering an accessible and flexible alternative for people who want clarity without continuous wear.
Clinically demonstrated and ready for scale
GlucoModicum has conducted extensive clinical studies (N>2000), showing accuracy in line with regulatory expectations for interstitial glucose monitoring. The core sampling and detection principles have also undergone independent peer review, supporting the scientific foundation of the device.
Sofio is engineered for mass manufacturability, with production partnerships established with leading global medical device contract manufacturers. The company is progressing through European regulatory pathways, with additional regulatory and commercial updates expected in 2026. GlucoModicum is also advancing its U.S. go-to-market and FDA pathway, with further details to be communicated in 2026.
Learn more: www.sofio.health.
About GlucoModicum
GlucoModicum is a Finnish medical technology company developing needle-free biosensing solutions powered by magnetohydrodynamic fluid sampling. The company’s mission is to make high-quality metabolic insights accessible to millions of people through clinically validated science and user-centered design. Sofio™ is the first commercial application of this technology.
Media Contact:
ICR Healthcare, Chris Welsh / Evi Useh
glucomodicum@icrhealthcare.com
www.sofio.health
www.glucomodicum.com
Attachment

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.